How to address the complexity of products being studied for multiple indications in multiple drug delivery options
Prepare for the multiple scenarios of the product being studied.
Early on, start examining the potential life cycle of the product. With a renewed focus on patient care, what is the best delivery method for the patient? Will it be self-administered, in a physician’s office, or administered in a hospital setting? It is important to think of what the product looks like from the start and what it should look like in the future for the best patient experience. A product may start with one presentation and end in an entirely different presentation because that is what is determined to be the best experience for the patient.
Considering all the different material and component options with component suppliers and your manufacturer at the beginning also help. For example, do your suppliers have long-lead times, and can they provide flexibility if you need to pivot as studies progress and your needs change? Does your manufacturer have vial, syringe, and cartridge capabilities, and do they have the equipment to scale up as studies progress? Is there a potential that the patient could self-administer the medication or even utilize an on-body system? Discussing with suppliers and manufacturers early on helps see the full picture of what’s available in the timeframe required to meet your product development goals.
Understand regulatory requirements for each indication and drug delivery platform.
Regulatory pathways continue to evolve as the pharma industry moves towards drug-device combination products to give patients a better experience. It is important to understand the regulatory pathway to approval and commercialization at the start of the project. Outside consultants should be considered for their regulatory expertise in combination products, as it is becoming increasingly more complicated to understand regulatory expectations. Regulatory experts can also help develop a strategy for each phase of product development.
Sharing knowledge and experiences while openly communicating potential drug delivery scenarios amongst all your suppliers, manufacturers, and consultants is necessary to ensure drug products safely and effectively impact patients. Be transparent throughout product development to ensure your partners have recent regulatory experience, especially with combination products.
Investigate multiple CDMOs and consider their external partnerships.
Working with CDMOs and their partners that have a range of experiences is immensely helpful in ensuring your product successfully navigates from clinical development to commercial manufacturing. Equipment capabilities can impact primary packaging component selection, and it is important your CDMO’s capabilities align with all your product development scenarios and can support scale-up and distribution. CDMOs that have invested in newer facilities will have the latest innovative technology, which is especially important with sterile filling and finishing to ensure the highest quality. Experience, capabilities, equipment, quality record, and partnerships are all key considerations when deciding who to trust to manufacture your drug product.
CDMOs that have strong partnerships with equipment manufacturers receive responses quickly and work together easily to come up with solutions for your product as it evolves during development and commercialization. Strong relationships aid in product discussions to confirm you have the right drug container for your product and avoid issues during production. Established supplier partnerships also help get components when needed or provide alternate options based on availability and timelines.
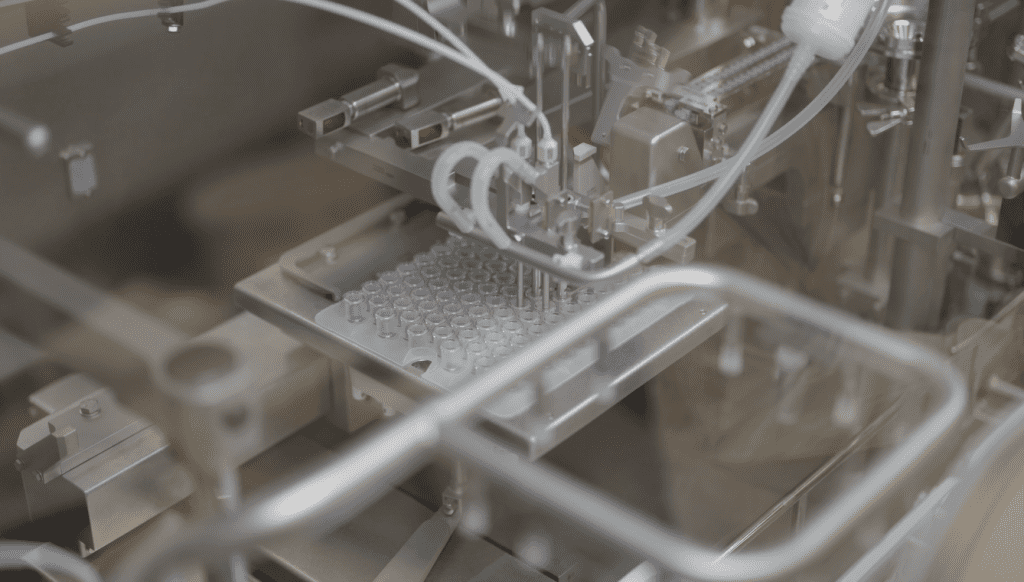
Consider modular sterile filling technology to provide flexibility.
To address the potential for multiple drug delivery scenarios during product development, consider modular sterile filling technology. Modular filling technology’s innovative design supports manufacturing in multiple formats using the same filling line, all with state-of-the-art isolator technology. As you need to go from a vial to a syringe or a cartridge, modular technology is set up to seamlessly assist with that transition.
Modular filling equipment is beneficial for product development and the manufacture of clinical materials because of its ability to run small batches in one container, then switch modules to a different component. Another great benefit of modular technology is the flexibility to scale up during commercialization when the final drug delivery and component selection are still in question. This is especially important with biologics, as modular technology can make it easier when looking for the best way to deliver the therapeutic to the patient.
Stay launch ready.
Plan for success and stay launch ready with Grand River Aseptic Manufacturing (GRAM). GRAM has three sterile filling lines, with a fourth on the way, all with Bausch+Ströbel filling equipment and SKAN isolator technology. All of GRAM’s sterile filling lines utilize identical filling and isolator technology, which provides clients with the ultimate versatility and flexibility to seamlessly scale their products as needed to succeed in the marketplace. Specifically related to modular technology, GRAM’s equipment includes the VarioSys. GRAM, Bausch+Ströbel, and SKAN partnered up on a video series called VarioSys World that showcases key attributes and benefits of modular technology.
Contact our team today by email at grambd@grandriverasepticmfg.com.
