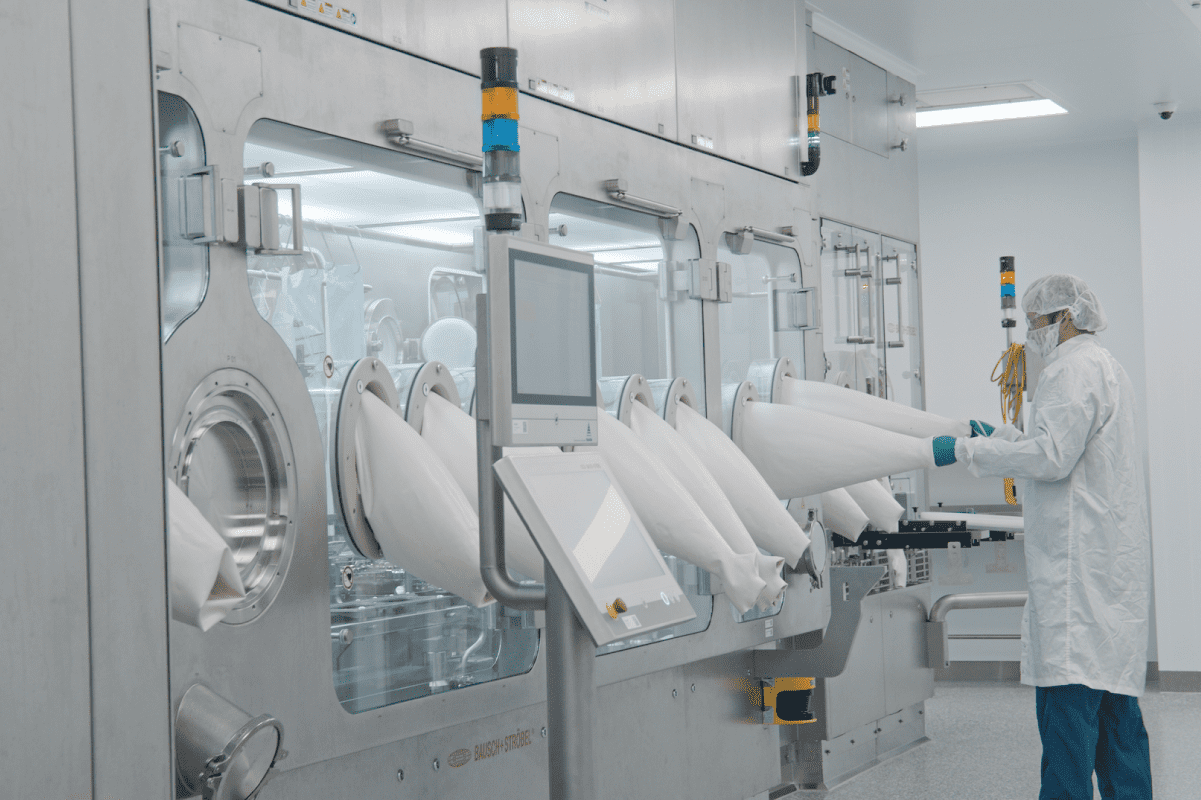
Isolators: What To Know
Isolator technology is a critical component in preserving sterile manufacturing and aseptic fill/finish of pharmaceutical products, especially in multi-product facilities. Isolators allow pharmaceutical experts to manufacture drug products with the highest regard for safety and quality.
Benefits of Isolator Technology
- Removes the most significant source of contamination by eliminating direct interventions with gowned employees
- Provides a high sterility assurance level (SAL)
- Allows the aseptic environment to be positive pressure or negative, have humidity control, oxygen control, and use unidirectional airflow
- Increases operator safety and reduces potential product exposure by isolating operators from the product
Common Client Questions
How does isolator technology work?
Isolators create an airtight barrier around equipment such as an injectable fill line and capping machine. The barrier protects operators from product and product from operators who perform tasks through glove ports, ultimately eliminating direct human contact with the product.
Why does the decontamination cycle matter?
Isolators have integrated decontamination systems that allow the process to take place quicker, more efficiently, and validates the decontamination cycle. Newer isolators use SKANFOG technology which is an advanced disinfection system that reduces process time by several hours. Implementing a replicable, routine cycle is critical to optimal isolator function.
Why should I use isolators for my product?
With the avoidance of cross-contamination as important as ever, isolators comply with sterile manufacturing regulatory requirements (FDA, EU) and are considered the safest method for maintaining high sterility levels and reducing risk of impurity.
What is a risk assessment?
A risk assessment identifies areas of risk for cross-contamination in facilities utilized for multiple products. The assessment examines transfers in and out of isolator areas and how these areas impact sterility and containment methods. Potential modes for cross-contamination and possible causes are assessed against current controls, probability of occurrence, and severity of consequences covering all GMP operations and incorporating EH&S (environment health and safety) requirements.
How do I prepare for isolator technology?
First, understand which products require isolator technology. Ask your CDMO for their risk assessment approach, experience using isolator technology, and what the support is like from their OEM (original equipment manufacturer). Strong relationships play a key role in successfully supporting your product.
Speak with an expert and get started on your next project.